Tackling High Medical Need Cancers with the Power of Large-Scale Genomics
5 Dec 2024
What does “high medical need“ mean?
The term "high medical need" in the context of cancer, encompasses a spectrum of challenges faced by patients and clinicians alike. It often refers to cancers with limited or no effective treatment options, where innovative solutions are urgently needed. It can also describe cancers that are notoriously difficult to diagnose—whether due to subtle early symptoms, late-stage detection, or the lack of reliable diagnostic markers.
Even in cancer types that are extensively studied and have multiple treatment options, the battle is far from over, as many patients can experience treatment resistance over the course of their therapy. This issue places these cancers firmly under the umbrella of high medical need, particularly in advanced stages where treatment options become increasingly limited.
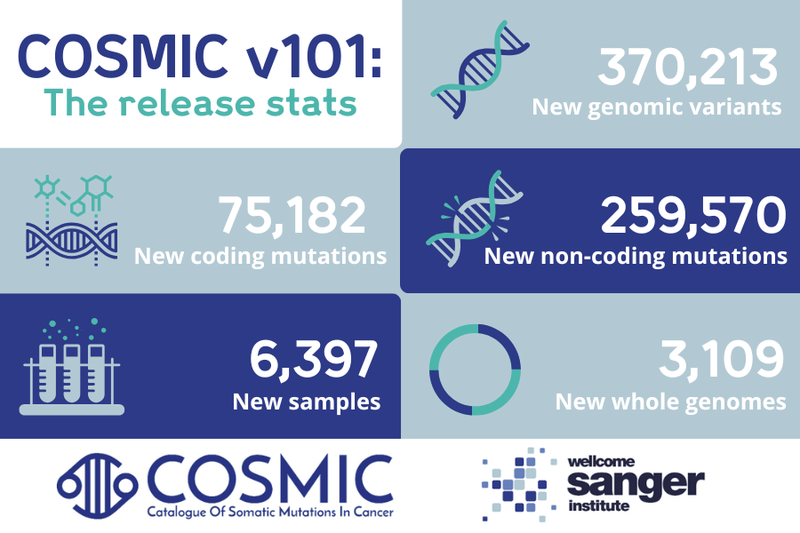
Across these diverse scenarios, one thing is clear: a comprehensive understanding of these unique and often rare diseases is essential. It ensures that no patient is left behind, whether their need arises from a lack of initial treatment options, challenges in diagnosis, or resistance to available therapies. In this blog post, we delve into COSMIC v101, and how our unparalleled curation empowers researchers, clinicians and beyond when addressing the genetic mechanisms behind every stage of these cancers.
Difficulty in diagnosis
Rare and molecularly diverse, Biliary tract cancer (BTC) is challenging to diagnose, and with limited therapeutic options available, long-term prognoses are poor. BTC is a prime example of a high medical need cancer featured in COSMIC v101. One particular study in this release used integrative clinical sequencing to analyse advanced BTC tumours from 124 patients who had progressed on standard therapies between 2011 and 2020 (PMID: 37267699).
This study identified actionable somatic and germline genomic alterations in 43.5% of patients and potentially actionable alterations in 63.7%. Among patients who received matched targeted therapy based on these findings, median overall survival was 28.1 months—more than double that of patients who did not receive matched therapy (13.3 months) or those without actionable mutations (13.9 months; P < 0.01). Notably, recurrent FGFR2 activating mutations, novel FGFR2 fusion partners, and an association between KRAS and BRAF mutant tumours with high expression of the immune modulatory protein NT5E (CD73) were discovered, presenting new diagnostic and therapeutic opportunities.
This study underscores the critical role of integrative sequencing in defining targeted therapy options for BTC. By providing molecular insights that directly impact patient care, it highlights the value of comprehensive genomic profiling for rare cancers, paving the way for improved diagnostics, treatments, and outcomes.
Rare exposures
Rare cancers take many forms ranging from subtypes of more common cancers to rare environmental causes. One commonality though, is how often they are understudied and underfunded in research, making them critical to address. These tumour types frequently lack the large-scale genomic studies that inform better diagnostics and treatments. One such example, included in COSMIC v101, is a systematic screen paper on Chernobyl radiation-related genomic alterations in papillary thyroid cancer (PMID: 33888599).
This study analysed the impact of the 1986 Chernobyl nuclear disaster by performing Whole Genome Sequencing (WGS) on 383 samples. Of these, 305 samples were from patients exposed as children to ionising radiation from the accident, while 78 samples were from individuals born after 1986. WGS revealed a total of 318,907 somatic mutations, all of which have been added to COSMIC. This dataset includes 315,022 non-coding variants and 3,885 coding variants, providing invaluable insights into this rare but critical cancer type.
Over half the designated driver events (59%) were mutations. The most commonly mutated gene was BRAF, accounting for 45.2% of the cases, followed by the RAS family genes—NRAS (4.7%), HRAS (3.5%), and KRAS (2.1%). The majority of BRAF mutations were the canonical BRAF V600E mutations, which are well-known in sporadic papillary thyroid cancer. In other cases, fusion events drove tumorigenesis, with fusion drivers being more common among those exposed to higher radiation doses and those diagnosed at a younger age.
They concluded that thyroid tumorigenesis following radiation exposure results from DNA double-strand breaks in the genome that have an impact on key thyroid cell growth and differentiation genes. These findings not only enhance our understanding of radiation-induced thyroid cancer but also underscore the importance of studying rare cancer types. By including large-scale data like this in COSMIC v101, we aim to provide researchers with the tools needed to drive discoveries in cancers with high medical need, regardless of their rarity.
Empowering research
This urgent need to characterise the vast range of cancers of high medical need was a key motivation behind our curation focus for COSMIC v101. Based on our users' valuable feedback, we prioritised studies focused on Whole Exome Sequencing, Whole Genome Sequencing, and NGS panel data, to provide a comprehensive view of these diseases.
Rarer tumour studies, in particular, present unique challenges, requiring curators to identify and catalogue new tumour types—a process that is both time-consuming and often under-prioritised within the cancer research community. For this release, however, our dedicated team of curators tackled this discrepancy head on, committing themselves to accumulating large-scale studies from multiple rarer tumour types, layered with actionable insights. Through this curation, we provide researchers with a comprehensive source of gold-standard data they can trust, empowering their research and transforming the future of healthcare.